Development of the Instrument for Cold Ion Spectroscopy
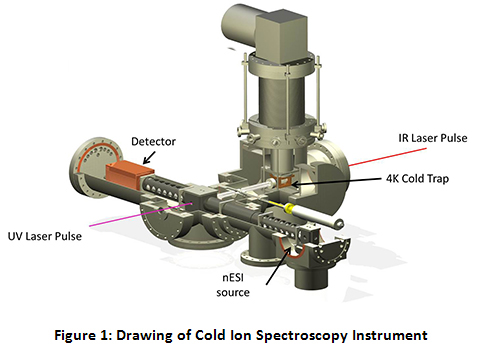
The Instrument for Cold Ion Spectroscopy at Purdue University (ICIS@Purdue) is an ambitious project that was undertaken in a collaborative effort that combines the expertise of the research groups of Professors Scott McLuckey and Timothy Zwier. The goal of this instrument is to combine the analytical power of a multi-source triple quadrupole tandem mass spectrometer with the spectroscopic power of cold, high-resolution single and double resonance UV/Vis/IR laser techniques. See Figure 1 for a cutaway drawing of the instrument.
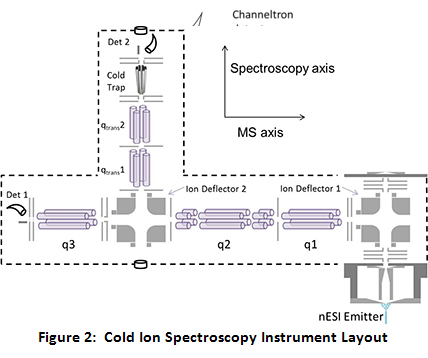
Specifically, the instrument consists of two orthogonal axes, a mass spectrometric axis and a spectroscopic axis. The mass spectrometric axis consists of three quadrupoles. The second and third quadrupoles (q2 and q3) have been modified to be operated as linear ion traps (LITs). After ions are generated via nano-electrospray ionization (nESI) and transported through a home-built miniature ion funnel, they are turned by the first DC quadrupole ion deflector, transported through q1 and trapped in q2. While in q2, the parent ion of interest is isolated via a notched chirp broadband waveform. Because q2 is set up as a linear ion trap with dipolar capabilities, the trapped, isolated ions can be further manipulated via applying resonant AC waveforms (“tickles”). After q2, the isolated parent ions pass through a second ion deflector where they can either continue straight into q3 or be turned down onto the spectroscopy axis.
If the ions are turned onto the spectroscopy axis, they pass through two transport quadrupoles into the ultra-high vacuum segment of the instrument. After the second transport quad, the ions are introduced into the cold trap. Throughout the course of this project, there have been a variety of cold traps utilized, including a heavily modified Paul Trap, a 22-pole ion trap, and currently an octopole cold trap. While the cold trap is typically held at 5 K by a closed cycle helium cryostat, a 50 W cartridge heater coupled with a temperature controller allows for precision temperature control of the cold trap from 5 K to 120 K for temperature-dependent studies. To trap and cool the ions, a pulse of ultra-pure He (99.9999%) is introduced directly to the trap 1–3 ms before the ions are introduced. The He atoms are cooled to near the trap temperature via collisions with the copper trap enclosure. Collisions between the parent ions and the cold He buffer gas cool the ions to below 10 K. At these temperatures, the ion population collapses into the lowest energy conformations, at their ground vibrational state, allowing for the application of high resolution spectroscopy, as currently employed in the Zwier lab for interrogation of neutrals.
After the lasers have interacted with the ions, the ions will be brought back out through the transport quads and turned into the q3 linear ion trap. In q3 a waveform generator applies a broadband waveform that serves to eject any parent ions remaining after irradiation, leaving only photofragments in q3. At this point, the photofragments can be mass analyzed via mass selective axial ejection (MSAE) or can be dumped as an ion packet into the channeltron detector by applying an attractive potential on the q3 exit lens. The latter method results in much smaller ion losses during detection and also allows for the collection of a photofragmentation spectrum that is the sum of the ion signal from all of the individual photofragment mass channels. The photofragment ion intensity is plotted as a function of wavelength to give the spectra. Comparison of the IR spectra to high level quantum mechanical calculations lends insights as to the structure of the ions. See Figure 2 for a schematic drawing of the instrument layout.
Due to the modest budget and highly ambitious nature of this instrumentation project, we have made extensive use of electronics and hardware which were designed and built by the Amy Instrumentation Facility. A prime example is the high power, high precision RF generators used on q2 and q3. There are no commercially available RF generators available for powering home built linear ion trap instruments that allow the range of operating conditions we desired. These supplies have been shown to be capable of >1000 Dalton mass range in routine operation for >50% duty cycle. The facility also built low power, vacuum-tube-based RF generators, which have shown excellent durability and adjustability, to drive transport quads and the in-house designed/built ion funnel. Additionally, the facility built a transistor-based RF generator to drive the cold trap. The electronics associated with driving our pulse valve are based on a proven Amy Facility design. The electronics associated with high voltage switching were originally based on a proven relay-based Amy Facility design; and, they have now been modified to a novel FET-based Amy Facility design.
Much of the vacuum system, support structure and vacuum-compatible components were also made in the Amy Facility’s Precision Machine Shop. Specifically, the 22-pole ion trap, transport quads, ion funnel, cold trap housing, octopole ion trap and several mounts for the system were made custom for us, in addition to the modification of flanges and feedthroughs to suit our needs. The parts produced were all tested for high vacuum integrity, taking advantage of the facility staff to receive one-on-one guidance on proper high vacuum material selection and construction techniques, saving us time and energy.
Construction of the instrument was completed in the spring of 2012. At this time, the Amy Facility was instrumental in helping to develop the LabVIEW software necessary for integrating the instrument and the laser systems required for the spectroscopy experiments. Since this time, the instrument has been successfully taking cold, high-resolution UV and IR spectroscopy of biologically relevant species. Additionally, there have been a variety of on-going instrument modifications and improvements undertaken with the help of the Amy Facility. Future instrumentation projects for this instrument include the continued development of RF supplies, the addition of a Time-of-Flight mass analyzer, and the modification of the q2 LIT electronics to allow for various ion-ion reactions, which Prof. McLuckey’s group has explored extensively.
A key benefit of having the Amy Facility in-house is flexibility. When modifications are necessary to accommodate a new capability or experiment, the knowledge is easily available to us and any modifications can be made quickly. This increases productivity in the long run and increases the probability that everything will operate correctly the first time. The collection of specialties available in the Amy Facility – vacuum, cryogenics, rf electronics, machining, programming – allow our projects to be fully vetted prior to actually implementing any designs.
Sources:
- Dr. R. Michael Everly, Amy Instrumentation Facility Director
- Rob Oglesbee, Amy Facility Senior Instrumentation Specialist
- Zachary Davis, Zwier Group Graduate Student
- James Redwine, McLuckey Group Graduate Student
- Jim Zimmerman, Amy Facility Senior Instrumentation Specialist
- Nicole Burke, McLuckey/Zwier Group Graduate Student
Video:
- Steve Scherer, Chemistry Communications
Publications:
- Nicole L. Burke, James G. Redwine, Jacob C. Dean, Scott A. McLuckey, Timothy S. Zwier, Int. J. Mass Spectrom., 2015, 378, 196-205. “UV and IR spectroscopy of cold protonated leucine enkephalin.”
- Jacob C. Dean, Nicole L. Burke, John R. Hopkins, James G. Redwine, P. Veeraraghavan Ramachandran, Scott A. McLuckey, Timothy S. Zwier, J. Phys. Chem. A, 2015, 119, 1917-1932. “UV photofragmentation and IR spectroscopy of cold, G-type β-O-4 and β-β dilignol-alkali metal complexes: structure and linkage-dependent photofragmentation.”
- James G. Redwine, Zachary A. Davis, Nicole L. Burke, Rob A. Oglesbee, Scott A. McLuckey, Timothy S. Zwier, Int. J. Mass Spectrom., 2013, 348, 9-14. “A novel ion trap based tandem mass spectrometer for the spectroscopic study of cold gas phase polyatomic ions.”
Professor Scott McLuckey describes why the instrument is unique and how the Amy Instrumentation Facility enabled the project.
Graduate Student Zachary Davis explains the expertise utilized in the instrument.
Graduate Student James Redwine describes the advantages the Amy Instrumentation Facility provides graduate researchers.
Senior Instrumentation Specialist Rob Oglesbee explains the advantages of having on-site engineers and technicians.
- A Look Inside the Amy Facility
- 8-Channel RF Signal Generator
- Apple Pencil Charger
- Photochemical Reactor
- RCF Controller
- High Bandwidth 16-Channel PMT Amplifier
- Microsecond Raman imaging might probe cells, organs for disease
- O'Buoy Project
- ALAR
- Argos Data Collector
- Vertical Air Profiler
- Triboluminescence
- Flow Reactor
- TRAC
- E-Beam Project
- Coiled Tubing Reactor
- Carbon Fiber Tubing
- Cryogenic Cooling Stage
- Photomultiplier Tube Power Supply
- Cold Ion Spectroscopy
- Mass Spec Solids Probe
- Spherical Void Electrodynamic Levitator
- Linear Rail Fatigue Tester
- Florescence Imaging System
- Pulse Stretching Amplifier
- Modular Low-Cost Photoreactor Chamber
- Hot Plate Photoreactor



